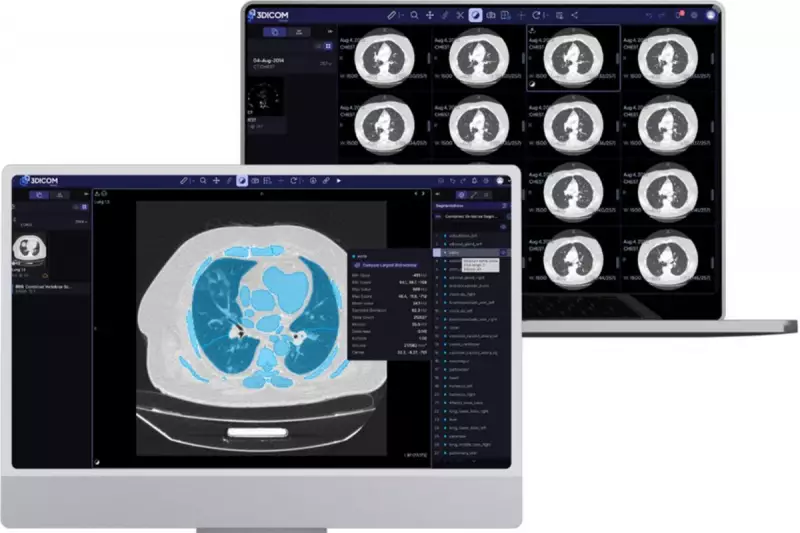
In a significant move for Australia's medical technology sector, Perth-based Singular Health Group has formally lodged a 510(k) notification with the powerful US Food and Drug Administration (FDA). The submission, made on Friday, 28 November 2025, seeks clearance to launch the company's innovative cloud-based "3DICOM" medical imaging platform into the massive United States healthcare market.
A 90-Day Countdown to Market Transformation
The company has confirmed that a 90-day review clock is now officially ticking. A successful outcome would mark the culmination of Singular's cloud commercialisation strategy and significantly accelerate its US technology expansion. This application is considered low-risk because it represents a browser-only enhancement to the company's next-generation 3D medical imaging software, which already received FDA clearance back in 2022.
This new cloud platform takes all the groundbreaking functionality of the original 3DICOM Viewer and migrates it entirely to the cloud. The service promises to give clinicians the ability to access fully interactive 3D reconstructions of patient scans from any web browser, anywhere. This is a monumental leap forward from the clunky, single-slice images that have long been a limitation of older technology.
Enhanced Capabilities and Future-Ready Architecture
The Cloud release introduces several key upgrades. It now includes native support for X-ray and ultrasound imaging, building upon the existing capabilities for CT, MRI, and PET scans. Furthermore, the platform features a completely redesigned user interface, shaped by direct feedback from practising Australian radiologists to ensure it meets real-world clinical needs.
Perhaps most strategically, the architecture has been constructed from the ground up to seamlessly integrate the AI modules of tomorrow. The platform will also host a comprehensive marketplace for medical image sharing, secure storage, and advanced analysis tools.
Shifting to a High-Demand Commercial Model
Securing a second FDA clearance would do more than just open the US market; it would fundamentally transform Singular Health's business model. The company would shift from one-off desktop software licences to a recurring cloud subscription service. This is precisely the kind of flexible, scalable structure that large US healthcare networks are actively seeking to purchase.
By moving to a zero-install, browser-based system, Singular directly addresses a major friction point that has hindered wider adoption in complex hospital IT environments. Removing this technical hurdle is expected to sharply accelerate uptake. This reinforces the company's core value proposition: slashing the billions wasted annually on duplicate scans by providing instant, universal access to prior imaging studies.
The FDA's target for reviewing a standard 510(k) application is approximately 90 days from the start of the review process, though this timeline can be paused if regulators require additional information.
"Submitting our 510(k) for 3DICOM MD™ Cloud is a major step in advancing Singular Health’s regulatory pathway for the next-generation of image visualisation and collaboration applications," stated Singular Health chief quality officer Andre Rocha. "This submission reflects a rigorous quality and risk-management process, and positions the Company to deliver a clinically enhanced, cloud-based platform aligned with U.S. regulatory expectations."
With cash reserves comfortably exceeding $10 million and the initial rollout of 1,000 licences already in motion, an FDA green light for the cloud-based system is poised to turbocharge the company's commercial expansion and solidify its position as a global leader in medical imaging technology.





